pH value
Definition pH value
The pH value is a measure of the acidic or alkaline character of a solution. It influences many chemical and biological processes. “pH” is derived from the Latin pondus hydrogenii (weight of hydrogen) or also from the Latin potentia hydrogenii (effectiveness of hydrogen) and is probably the most common measurand in analytics.
The pH value is generally given on a numerical scale of 0-14. Here, the value 7 stands for neutrality. The numbers on the scale increase with increasing alkalinity, while the numbers on the scale decrease with increasing acidity.
Each unit of change is equal to a tenfold change in acidity or alkalinity. The pH value is also equal to the negative logarithm of the hydrogen ion concentration or hydrogen ion activity.
The pH value is of outstanding importance in water and environmental analysis, as it is in almost all areas of industry. Whether the quality of a cheese in a dairy is right, the water in a drinking water supply causes corrosion damage or the precipitation in a treatment plant for electroplating wastewater is optimal for heavy metal ions depends, among other things, on the pH value.
The pH value is therefore about hydrogen or, more precisely, about hydrogen ions. In aqueous solutions, hydrogen ions are formed during the dissociation of acid or water molecules. Pure water dissociates as hydrogen ions (H+) and hydroxide ions (OH-).
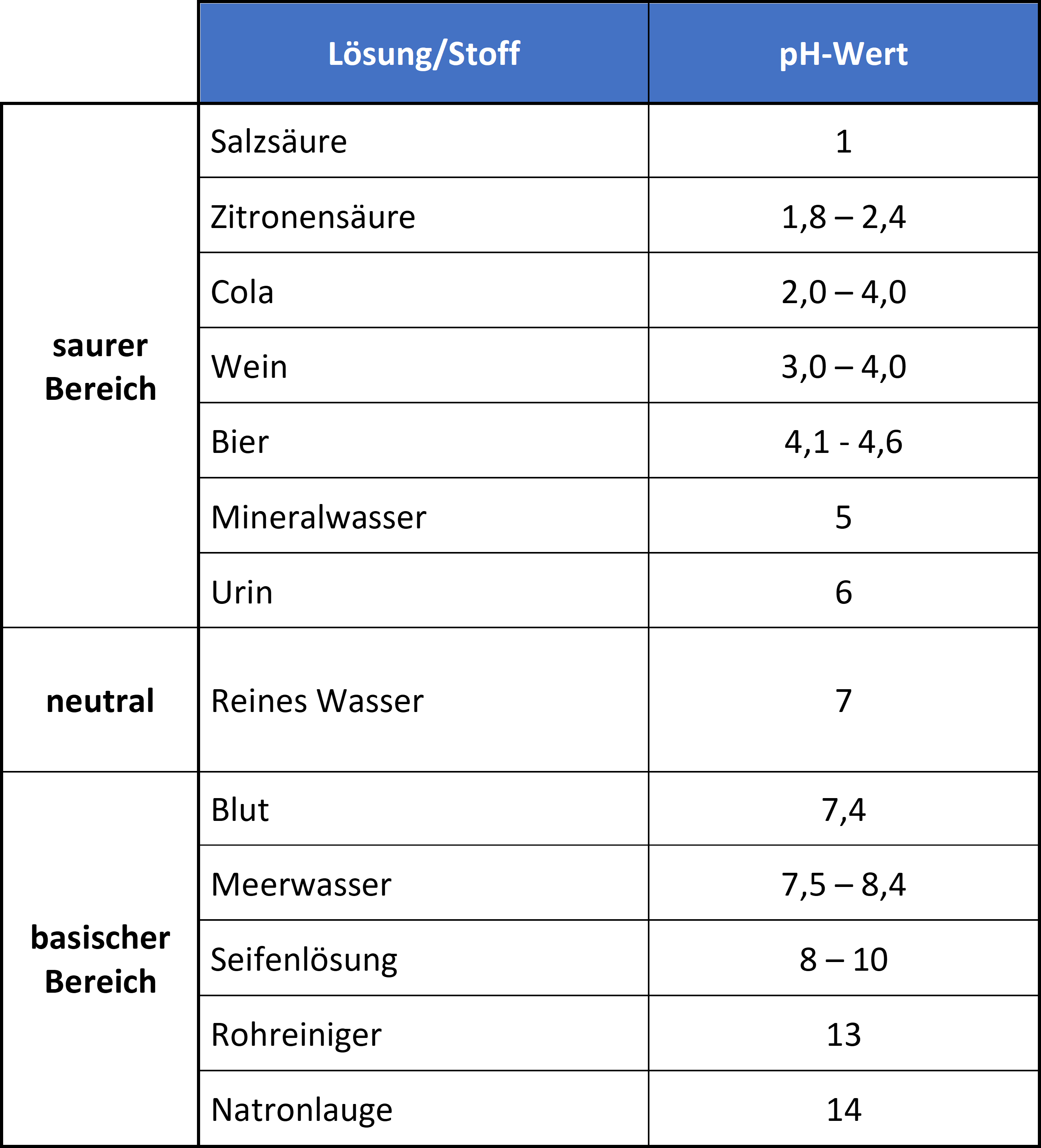
Acidic are solutions with a pH value lower than 7. Consequently, they contain more hydrogen ions than hydroxide ions. Neutral are solutions with a pH value of 7 and contain equal amounts of hydrogen and hydroxide ions (the exact neutral point depends on the temperature). Basic are solutions with a pH greater than 7 and contain fewer hydrogen ions than hydroxide ions.
There are the most different possibilities to measure the pH value: colorimetrically, photometrically or electrochemically. Different measurement methods can lead to different measurement results, which are all correct in principle. So that no confusion arises from this fact, national and international standardization stipulates that the pH value is measured electrochemically with a glass electrode measuring chain.
The sensor for the measurement is the pH electrode. It consists of two electrochemical half-elements, which are also referred to as the measuring and reference electrodes. At the measuring electrode, the hydrogen ions form a potential that depends on the pH value of the measured solution. The potential of the reference electrode remains independent of the pH value and is constant. The difference between the two potentials determines the electrical signal of the sensor, the so-called electrode voltage. This then determines the pH value.
The glass electrode is the most powerful sensor for pH measurement. Its operating range covers practically the entire pH range. Special membrane glasses are only required for strongly alkaline solutions. The glass electrode is of good resistance. The pH electrodes can last for several years and can be used in most measuring media.
The pH-sensitive part of the combination electrode consists of special glass mixtures. There are different types of glass for different applications. Since membrane glass is “transparent” regardless of the type, the manufacturers color the glasses with harmless additives for better differentiation.
The reference electrode complements the pH electrodes to the measuring chain. Their design and condition have a significant influence on the reliability of the measured values and the required maintenance effort. The most widely used is the silver/silver chloride electrode (Ag/AgCl). This reference electrode has been well proven and accepted for most applications. Not or no longer so frequently used are, for example, calomel, copper/copper iodide and thalamide reference electrodes.
The term measuring device encompasses the complete instrumentation used for pH measurement, consisting of:
- pH transmitter: pH and reference electrode or pH combination electrode
- immersion or flow-through fitting
- shielded measuring cable
- Transmitter/controller (mV meter)
The electrolyte and the diaphragm must be matched to each other depending on the application. For highly contaminating liquids, such as waste water, suspensions or emulsions, contamination-insensitive diaphragms are required, e.g. annular gap or ground joint. A solidified electrolyte, gel or polymer reduces the electrolyte outflow and thus the maintenance effort.
Electrolyte solutions are more suitable for optically pure waters, such as swimming pool and drinking water. Due to the lower viscosity, finer-pored diaphragms, e.g. ceramic or glass fiber diaphragms, are necessary here.
For many applications, the electrolyte must contain no silver ions or as few as possible. Solutions containing sulfite and low-salt water form poorly soluble silver compounds in the diaphragm and can lead to a disturbance of the pH measurement.
To obtain flawless transmission of the measurement signal, only special coaxial cables are used in pH measurement technology. They establish the electrical connection between the sensor and the transmitter. The pH cables have a special structure. In addition to a copper shield, there is a semiconducting layer. Commercially available antenna or computer cables are unsuitable. Due to the high impedance of the pH electrode, the cables must not be routed via terminals. In addition, the cable length must be kept as short as possible because of the need for electrode calibration.
The transducer has, among other things, the task of converting the high-impedance voltage signal of the pH electrode to the pH scale and making these values available again as a display and/or standard signal. The transmitter usually contains a calibration routine for electrode adjustment with buffer solutions. Frequently, the transmitters are also designed as controllers at the same time, which can, for example, carry out the dosing of acids and alkalis for pH value correction. In addition, the transmitters take the media temperature into account, either through manual input options or through a separate measuring input for the temperature sensor.
Electrochemical sensors are sensitive measuring devices. In order to be able to optimally integrate them into the process on the one hand and to protect them from mechanical damage on the other, the use of appropriate fittings is necessary. Optional accessories also prevent the sensors from “running dry” when the tank is drained and the sensor is destroyed. For this reason, pH sensors must never be stored “dry”.
After the selection of an optimal measuring device, it must be put into operation. Commissioning includes not only the installation of the measuring device, but also the selection of the correct measuring location. The measuring device always displays the pH value that prevails at the measurement location. Specifications for the choice of the measuring location are given, for example, by application-oriented standards and regulations, such as DIN 19643 for the measurement of swimming pool water or the M 256 leaflet of the Abwassertechnische Vereinigung.pH measurements are used in all areas of technology and environmental protection. Depending on the application, the measurement takes place in the laboratory, with a handheld device on site or continuously, e.g. in the process. The following practical applications of pH measurement can be mentioned as examples:
In the field of technical plant engineering, a wide variety of materials are used for piping systems, seals and plant components. Here, too, the pH value and the associated chemical resistance play a decisive role in material selection. The materials must be matched to the respective process and the substance to be used.
A classic application for pH value monitoring is heating water. VDI 2035-2 recommends a range between 8.2 and 9.5 for the pH value, provided that no aluminum materials are used.
Fittings made of copper alloys or components made of copper materials are often found in many circuits. For this reason, a pH value of 8.3 to 9.5 is recommended by experts, for example, for heating circulating water in saline operation. The background to this recommendation is, on the one hand, that from a pH value > 8.2, carbon dioxide can no longer be dissolved in the water. If carbon dioxide is present in dissolved form (carbonic acid) in the water, deposits can form on heat transfer surfaces in the form of poorly soluble iron carbonate (FeCO3, also known as “iron spar”). As a result of these deposits, cracks can form. Furthermore, investigations have shown that copper erosion increases significantly from a pH > 9.5. This leads to the upper limit of the named range for the recommended pH value.
When using iron, the pH range between 9 and 13 should be aimed for. Within this range, solid corrosion products form on the surface, which can exert a protective effect. For copper, a pH value > 7 is acceptable, but this must not exceed the value 11, as from here on it noticeably dissolves again. If ammonium is a component of the water, this process already starts at a pH value of 9.5.
If there are elements of aluminum in the circuit, the pH value must be limited to 8.5. Aluminum is passive at pH values between 4 and < 9. Above pH 9, the metal goes under hydrogen evolution, even in completely oxygen-tight/closed systems.
Thus, for systems containing aluminum components, the pH value without carbon dioxide in the system must be > 8.2 and otherwise not > 8.5 (max. 9.0).
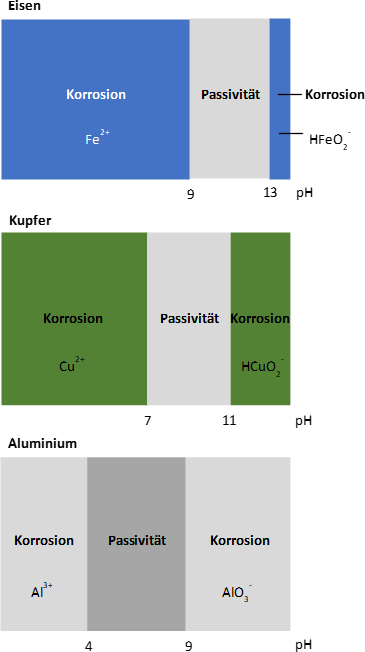
Another area of application is the use of galvanized tube bundles (typical in the use of ammonia refrigeration plants). Galvanized surfaces have not yet formed a protective layer directly after production. The zinc coating is very sensitive to higher pH values and must therefore not exceed 8.3 during the first six to twelve weeks after commissioning. If higher pH values are reached within this time, there is a risk of dezincification. The value may be raised to 8.8 when the matt zinc oxide layer has formed.
In summary, it can be stated that the pH value is a characteristic variable both for the manufacture of products and for the professional construction of plants and the continuous operation of such plants.




