Caustic soda dosing vs. membrane degassing
As a result of the increasing technologization of industrial manufacturing processes, there are increasingly stringent requirements for the purity of the water required. It is not unusual for ultra-pure water (demineralized water) to be required for individual process steps.
In order to produce this, different process stages are used, which build on each other and must therefore be coordinated. One of the most frequently used processes is reverse osmosis in combination with a downstream EDI. The combination of both processes enables the production of high-purity water.
The reverse osmosis process produces a CO² discharge due to the shift in the lime-carbonic acid equilibrium. In order to avoid a smooth process as well as damage to downstream components of the water treatment e.g. electrodeionization (EDI) and the risk of corrosion in metallic pipelines, the CO² has to be removed again. Proven processes such as caustic soda dosing and membrane degassing are available for this purpose.
In caustic soda dosing, a small amount of caustic soda is added to the water to reduce the concentration of carbon dioxide dissolved in the water. If larger quantities of carbon dioxide are to be neutralized, the sodium content in the water can thereby assume undesirably high values. Proper technical adjustment as well as monitoring and venting require technical expertise. When sodium hydroxide is used, a risk assessment must be drawn up for the workplace to ensure occupational safety, and proper storage of the chemicals must be ensured. When using the caustic soda dosing process, there is an increased safety hazard for the users.
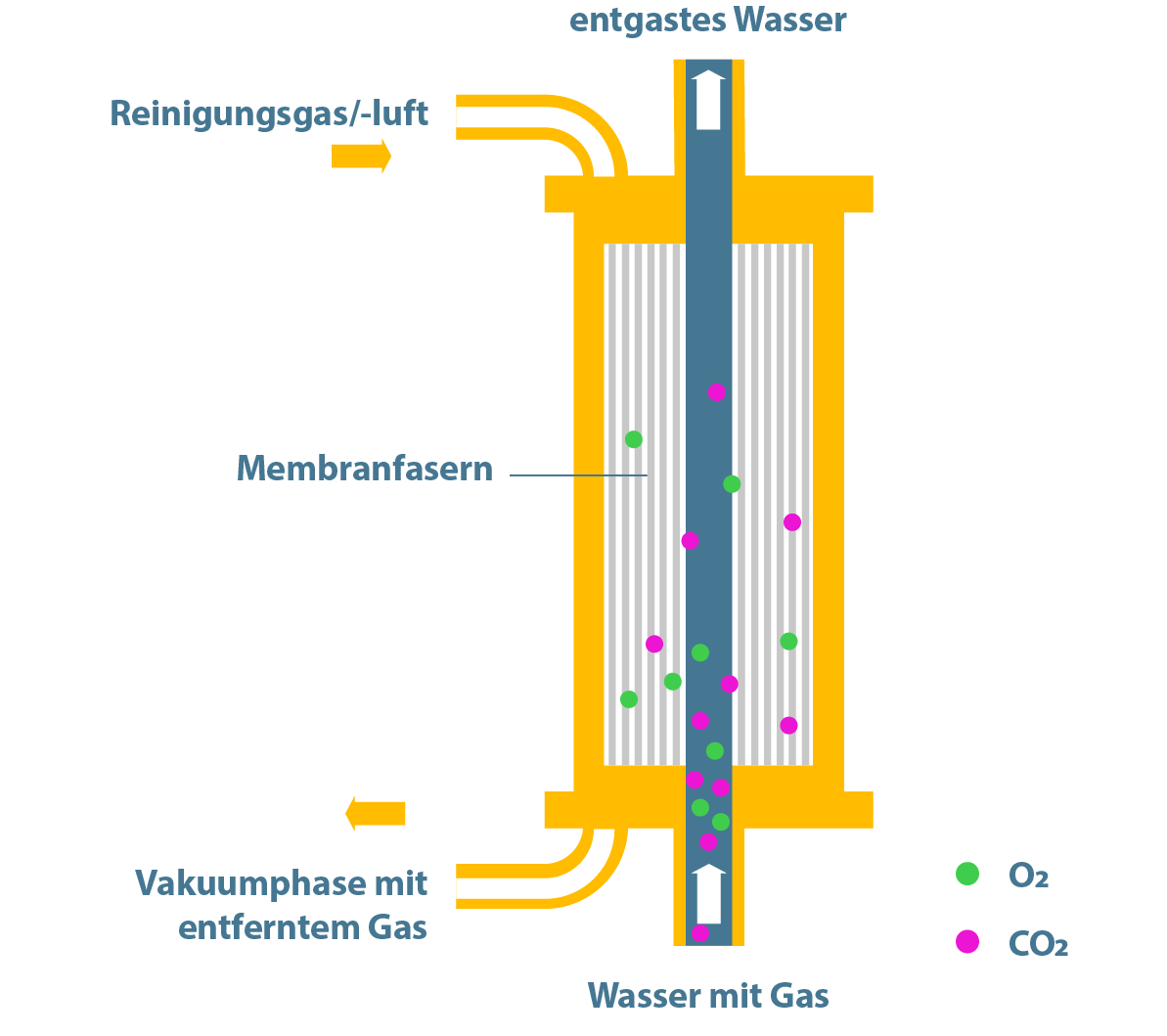
The membrane degassing process is available as a technically proven alternative to caustic soda dosing. The main advantage over caustic soda dosing is that no chemicals are used, so that there is no risk to employees from chemicals in terms of occupational safety.
Membrane degassing specifically removes free carbonic acid from the water. The modules consist of hydrophobic pore membranes. These are usually constructed as hollow fiber modules. Due to the different partial pressure, the gas diffuses through the membrane and the water is degassed.
Depending on the size of the plant and its capacity, the necessary partial pressure gradient is built up by means of a strip gas or vacuum, which is generated by vacuum blowers or via compressed air.
The operating principle of membrane degassing is essentially that air is drawn past an outer chamber of the modules in countercurrent to the water flow in the inner chamber. As a result, the free CO² is drawn from the water flow into the air flow and transported to the outside.
In summary, it can be stated that the membrane degassing process not only takes into account the protection of the downstream plant technology, but also offers significant advantages for the user against the background of technical support and occupational safety. Since no chemicals are used, this process should also be given priority in terms of sustainability.