Bioindicators for self-monitoring of sterilizers / autoclaves
Sterile processing in reprocessing units for medical devices in clinics, hospitals and medical care centers (MVZ) is a highly sensitive area. Steam sterilization is a fundamental element for success in ensuring the sterility of medical devices.
For these reasons, the inspection and accompanying validation of autoclaves in healthcare facilities is regulated in various standards:
- EN ISO 17665 – 1: 2006 – 11: In this international standard, the “Sterilization of health care products – Moist heat – Part 1: Requirements for the development, validation and control of the use of a sterilization process for medical devices” is determined. This standard is the successor regulation of the following standards DIN EN 554 (58946 – 6) and represents the state of the art regarding moist heat sterilization processes of medical devices.
- DIN EN 554: This specifies the “Sterilization of medical devices – validation and routine monitoring for moist heat sterilization”. Its main purpose is to ensure the quality of sterilization.
- DIN 58946-7: 2014 – 01: This regulates “Construction requirements as well as requirements for the equipment and operation of steam sterilizers in the healthcare sector”.
- DIN EN 285: 2016 – 05: This standard for “Sterilization – Steam sterilizers – Large sterilizers” specifies the essential performance requirements including the associated test methods.
- EN ISO 15883: Finally, this standard is a general “series of standards for washer-disinfectors” that relates to equipment requirements and validation of processes for reprocessing.
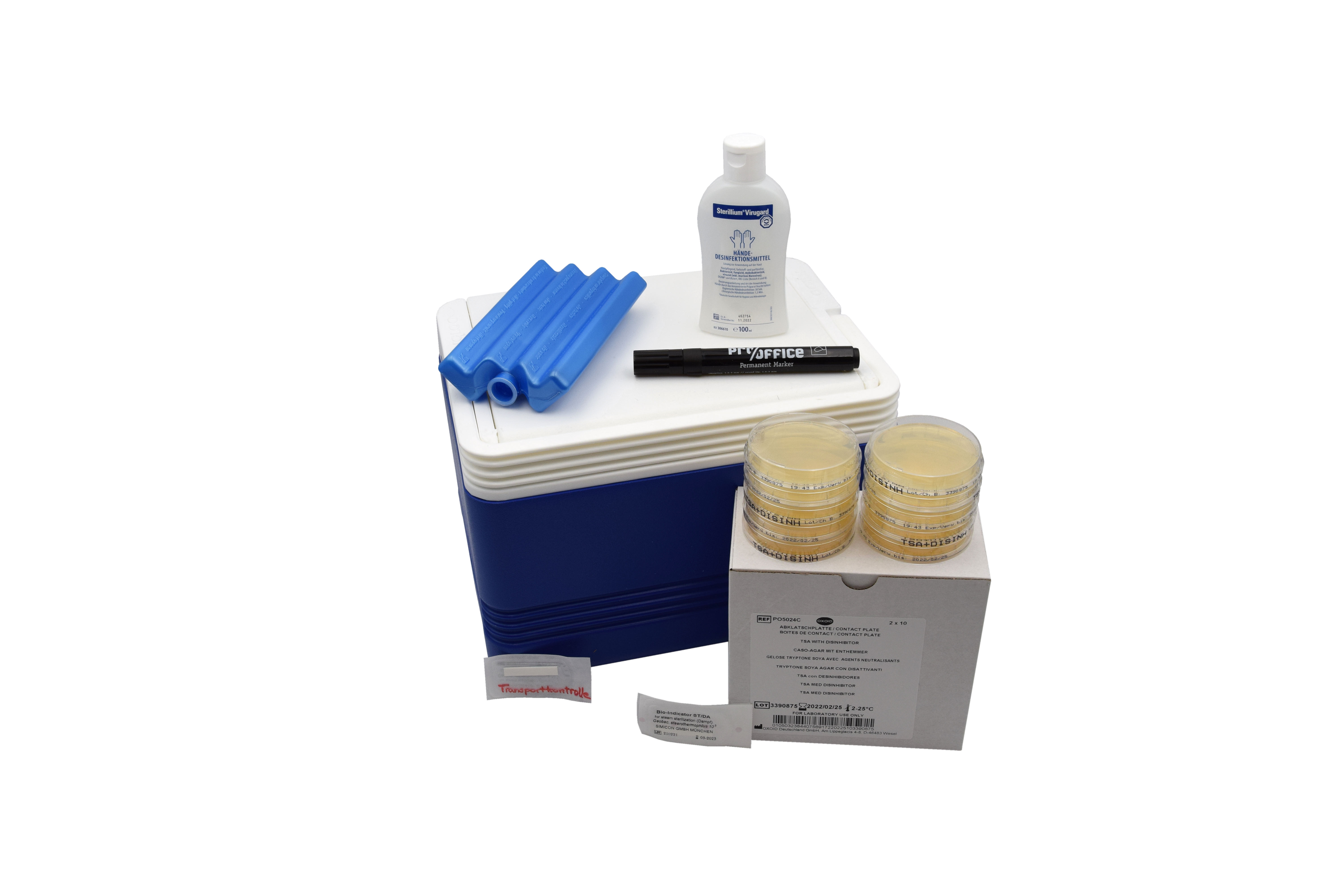
In this context, we used the Sterilization HyMo Box from Hohenstein Laboratories GmbH & Co. KG as a test method for validating the sterilization process in the AEMP as part of a process analysis in a clinic in the Düsseldorf administrative district.
With the aid of bioindicators added to the autoclaves or the autoclaving process, proof of successful germ elimination of the test germ “Geobacillus stearothermophilus” is provided. The sterilization process is thus biologically verified with the aid of the HyMo-Box.
The bioindicators are taken after sterilization, sent to the accredited Hohenstein laboratory and evaluated. The bioindicators are examined in the laboratory. If the test germ “Geobacillus stearothermophilus” grows up, the sterilization process is not valid.
In this case, further considerations of the sterilization process must be checked in follow-up with the operator with regard to the settings (e.g. temperature, programs, dosing …) as well as the functioning of the sterilizer.
Proof of successful germ elimination of the test germ in the laboratory, on the other hand, indicates a safe sterilization process.
The test result is sent to the operator as a report with evaluation by the Hohenstein Laboratory and has the character of a self-check to prove that the steam sterilization process was successful.
If sterilization is demonstrably successful, the process is safe in terms of reliably killing germs and the sterile goods passing through can be described as sterile. Microorganisms can only penetrate the sterile barrier if it is damaged.
The use of the bioindicators for sterilization is intended to help ensure the biological safety of the equipment. The sterilization box can be used in smaller facilities such as outpatient clinics and doctors’ offices as well as in hospitals and clinics.
Within the scope of our process analysis in AEMPs, the HyMo-Box is also used as an analytical tool, in addition to many other test procedures, in order to be able to make reliable statements about the process safety in the AEMP / sterilization.
The test procedure is carried out in cooperation with the accredited Hohenstein Laboratory. The biological safety check in the autoclave should be carried out at least every six months. The bioindicator test by means of HyMo-Box can be carried out independently and thus regularly by the responsible personnel with simple step-by-step instructions. The bioindicator test is a cost-effective tool for checking the sterilization process. The patient risk is considerably reduced by regular biological checks, possible OR postponements or cancellations are eliminated because the instrument sieve intended for use in the OR can be used.
The HyMo box also contains a set of plates that can be used to check the absence of germs on work surfaces that are used to prepare for sterilization, e.g., wrapping in sterilization packaging. With the supplied results of the surface germ counts, cleaning-disinfection plans can thus also be adapted and updated. In personnel hygiene, too, the plate can be a good tool for optimizing workflows in order to safeguard the entire sterilization process.